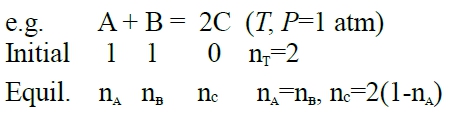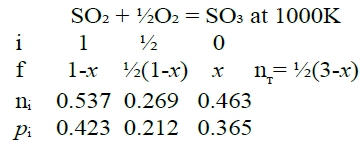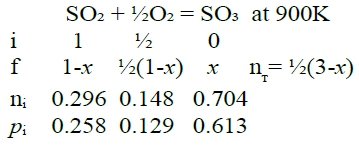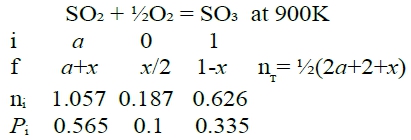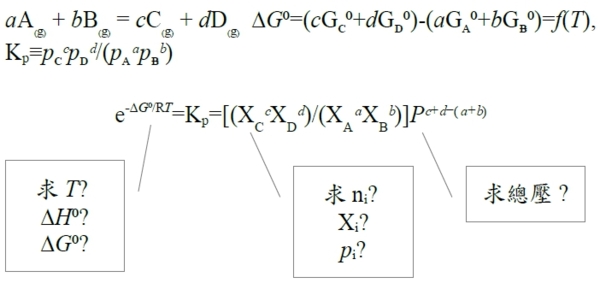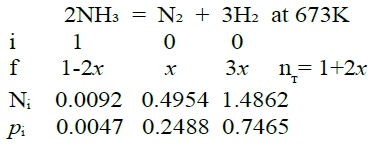字體:小 中 大
字體:小 中 大 |
|
|
|
| 2020/09/29 20:42:23瀏覽815|回應0|推薦0 | |
Ch. 11 chemical reaction 前面講溶解,只有相變化,無涉及化學反應,這章加上化學反應的氣體產物 A₍g₎+B₍g₎=2C₍g₎ ,∆Gᵢ 當成ideal gas mixture, pᵢ=XᵢP e.g. 2H₂+O₂=2H₂O
引進反應平衡常數,K, 因為用∆G推導時,利用K求nᵢ最快
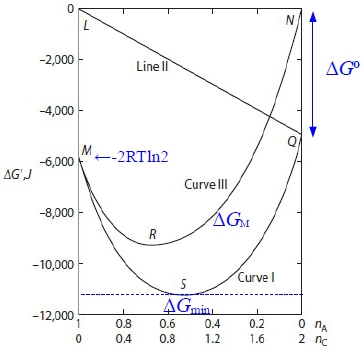
Gi=nᴬGᴀ⁰+nᴮGᴃ⁰= Gᴀ⁰+Gᴃ⁰, Gf=nᴬGᴀ+nᴮGᴃ+nᴄGᶜ=nᴬ(Gᴀ+Gᴃ-2Gᶜ)+2Gᶜ ∆G=Gf-Gi i.e. ∫Gᵢ⁰GᵢdGᵢ=∫₁pᵢRTdlnpᵢ, Gᵢ-Gᵢ⁰=ᵢRTlnpᵢ ⸫ Gᵢ=ᵢGᵢ⁰+RTlnXᵢ+RTlnP代入Gf式中, i=A, B, C, Xᴀ=Xᴃ=nᴬ/2, Xᶜ=(1-nᴬ) → Gf= nᴬ[Gᴀ⁰+RTlnXᴀ+RTlnP+Gᴃ⁰+RTlnXᴃ+RTlnP-2(Gᶜ⁰+RTlnXᶜ+RTlnP)]+2(Gᶜ⁰+RTlnXᶜ+RTlnP)=nᴬ(Gᴀ⁰+Gᴃ⁰-2Gᶜ⁰)+2RT[nᴬln(nᴬ/2)+(1-nᴬ)ln(1-nᴬ)]+2Gᶜ⁰ let 2Gᶜ⁰-(Gᴀ⁰+Gᴃ⁰)≡∆G⁰...standard Gibbs free energy change → ∆G=(1-nᴬ)∆G⁰+2RT[nᴬln(nᴬ/2)+(1-nᴬ)ln(1-nᴬ)], ∆Gᴿ=(1-nᴬ)∆G⁰ and ∆Gᴹ=2RT[nᴬln(nᴬ/2)+(1-nᴬ)ln(1-nᴬ)] equilibrtum: ∆G=∆Gₘᵢₙ → ∂∆G∕∂nᴬ=0 → nᴬ=?
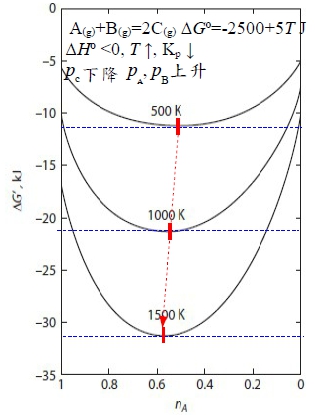
⸪Gi= Gᴀ⁰+Gᴃ⁰=const, ⸫∂∆G∕∂nᴬ=0 → ∂∆Gf∕∂nᴬ=0, Gf=nᴬGᴀ+nᴮGᴃ+nᶜGᶜ=nᴬ(Gᴀ+Gᴃ-2Gᶜ)+2Gᶜ ∂∆Gf∕∂nᴬ=Gᴀ+Gᴃ-2Gᶜ=Gᴀ⁰+ᵢRTlnpᴀ+ Gᴃ⁰+ᵢRTlnpᴃ-2(Gᶜ⁰+ᵢRTlnpᶜ)=0 → 2Gᶜ⁰-(Gᴀ⁰+Gᴃ⁰)=-RTln[pᶜ²/(pᴀpᴃ)], Kₚ≡pᶜ²/(pᴀpᴃ) → ∆G⁰=-RTlnKₚ, Kₚ=e-∆G⁰/RT def: A₍g₎+B₍g₎=2C₍g₎ , ∆G⁰=2Gᶜ⁰-(Gᴀ⁰+Gᴃ⁰) and Kₚ≡pᶜ²/(pᴀpᴃ) equil.: ∆G⁰=-RTlnKₚ (∂∆G∕∂nᴬ=0得到的結果), given: Gᴀ⁰, Gᴃ⁰, Gᶜ⁰ at T→ ∆G⁰ at T→ Kₚ=e-∆G⁰/RT e.g. Gᴀ⁰=Gᴃ⁰=0, Gᶜ⁰=-2500J A₍g₎+B₍g₎=2C₍g₎ A₍g₎+B₍g₎=2C₍g₎ 求 pᴀ, pᴃ, pᶜ ∆G⁰=-5000J,at T=500K, P=1 atm ⸫Kₚ=e-∆G⁰/RT=3.329=pᶜ²/(pᴀpᴃ)=(XᶜP)²/[(XᴀP)(XᴃP)]=(1-nᴬ)²/[(nᴬ/2)(nᴬ/2)] → 0.671nᴬ²-8nᴬ+4=0, nᴬ=0.523 pᴀ=pᴃ=0.261, pᶜ=0.477 §Effect of temperature on reaction equilibrium(T與Kₚ的關係) given ∆G⁰=-RTlnKₚ, 通常以反應熱∆H⁰表示 ∆G⁰↔∆H⁰ Gibbs-Helmholtz eq. [∂(∆G⁰/T)∕∂T]ₚ=-∆H⁰/T² ⸫[∂(-RlnKₚ)∕∂T]ₚ=-∆H⁰/T² → [∂(lnKₚ)∕∂T]ₚ=∆H⁰/RT² vant Hoff eq. If endothermic, ∆H⁰>0, 溫度增加, ∂(lnKₚ)∕∂T>0 → Kₚ隨之增加 ⸪Kₚ≡pᶜ²/(pᴀpᴃ) ⸫pᶜ上升 pᴀ, pB下降 A₍g₎+B₍g₎=2C₍g₎反應往右 Le Chatelier principle aA₍g₎ + bB₍g₎ = cC₍g₎ + dD₍g₎ ∆G⁰=(cGᶜ⁰+dGᴅ⁰)-(aGᴀ⁰+bGᴃ⁰)=f(T), Kₚ≡pᶜᶜpᴅᵈ/(pᴀᵃpᴃᵇ) equil. condition: ∆G⁰=-RTlnKₚ e.g. Given (T,P) → nᵢ=? Temp. Influence: vant Hoff eq. [∂(lnKₚ)∕∂T]ₚ=∆H⁰/RT² e.g. Cl₂₍g₎ → 2Cl₍g₎ ∆H⁰>0 Kₚ=pᴄₗ²/pᴄₗ² when T ↑, ∂T>0, ∂lnKₚ>0 → Kₚ ↑ ⸫pᴄₗ↑, pᴄₗ² ↓ 反應往右 §Influence of total pressure ⸪ Gᵢ⁰ defined at P=1 atm, ⸫∆G⁰與壓力無關 Kₚ=e-∆G⁰/RT=c₀ if T fixed ⸫Kₚ fixed也與壓力無關; 但改變總壓, pᵢ may change!! pᵢ=XᵢP → Kₚ= when a+b=c+d → Kₚ= Kₓ, nᵢ(Xᵢ) no change when a+b<c+d → c+d-(a+b)>0 ⸫P上升, Kₓ下降 ⸪Kₚ fixed, 因此Xᶜ, Xᴅ減少, Xᴀ, Xᴃ增加 總壓力增加,反應往左 當平衡時, ∆G⁰=-RTlnKₚ; ∆G=∆H-T∆S at fixed T, ∆Gₘᵢₙ需要∆H↓, ∆S↑兩者妥協
Gf=nᴬ(Gᴀ⁰+Gᴃ⁰-2Gᶜ⁰)+2RT[nᴬln(nᴬ/2)+(1-nᴬ)ln(1-nᴬ)]+2Gᶜ⁰ → ∆G=Gf-2Gᶜ⁰=-nᴬ∆G⁰+2RT[nᴬln(nᴬ/2)+(1-nᴬ)ln(1-nᴬ)]=-nᴬ∆H⁰+T{nᴬ∆S⁰+2R[nᴬln(nᴬ/2)+(1-nᴬ)ln(1-nᴬ)]} ⸫∆H=-nᴬ∆H⁰, ∆S=-{nᴬ∆S⁰+2R[nᴬln(nᴬ/2)+(1-nᴬ)ln(1-nᴬ)]}
Ex. SO₂₍g₎ + ½O₂₍g₎ = SO₃₍g₎ given: ∆G⁰=-94600+89.37T, ask nᵢ, pᵢ in equilibrium under T=1000K, P=1 atm, nSO₂⁰=1 mole, nO₂⁰=0.5 mole sol: T=1000K, ∆G⁰=-5230J Kₚ=e-∆G⁰/RT=1.876≡pSO₃/(pSO₂pO₂½)=[XSO₃/(XSO₂XO₂½)]∙P⁻½ → Kₚ²=[XSO₃²/(XSO₂²XO₂)]∙P⁻¹ → (1-PKₚ²)x³+(3PKₚ²-3)x²-3PKₚ²x+ PKₚ²=0 代入P=1, Kₚ=1.876 x=0.463
Q2: ask nᵢ, pᵢ in equilibrium at T=900K with the same condition. T=900K, ∆G⁰=-14167J Kₚ=e-∆G⁰/RT=6.64=pSO₃/(pSO₂pO₂½)=[XSO₃/(XSO₂XO₂½)]∙P⁻½ → Kₚ²=[XSO₃²/(XSO₂²XO₂)]∙P⁻¹ → (1-PKₚ²)x³+(3PKₚ²-3)x²-3PKₚ²x+ PKₚ²=0 代入P=1, Kₚ=6.64, x=0.704
Q3: ask nᵢ under equilibrium with P=10 atm at 1000K (1-PKₚ²)x³+(3PKₚ²-3)x²-3PKₚ²x+ PKₚ²=0 代入P=10, Kₚ=1.876 → x=0.686 nSO2= 0.314, nO2 = 0.157, nSO3= 0.686 → pSO₃=5.93 atm, pSO₂=2.71 atm, pO₂=1.36 atm check: ∆H⁰=-94600<0 exothermic T↓ [∂(lnKₚ)∕∂T]ₚ=∆H⁰/RT²<0, ⸪∂T<0 ⸫∂(lnKₚ)>0 → Kₚ=pSO₃/(pSO₂pO₂½)↑, pSO₃↑ pSO₂pO₂↓反應往右 P↑ Kₚ=[XSO₃/(XSO₂XO₂½)]∙P⁻½=c₀, ⸪P⁻½ ↓ ⸫XSO₃/(XSO₂XO₂½)↑ → XSO₃↑ XSO₂XO₂↓反應往右 §藉氣體混合來調節氧分壓 SO₂₍g₎ + ½O₂₍g₎ = SO₃₍g₎ at 1000K, 1 atm. Assuming pO₂=0.1 atm ask nSO2/nSO3=a=? under equil.
pO₂=XO₂P=x/(2a+2+x)=0.1, a=4.5x-1 Kₚ²=[XSO₃²/(XSO₂²XO₂)]∙P⁻¹=(1-x)²(2a+2+x)/(a+x)²xP=1.876 代入P=1, a=4.5x-1 → 96.45x²-18.709x-6.481=0, x=0.374 → a=0.683 Q: when pO₂=max. a=? 氧由SO₃ 分解而來, nSO2⁰=0 ⸫a=0 H₂/H₂O mixure at T=2000K, P=1 atm given: H₂₍g₎ + ½O₂₍g₎ = H₂O₍g₎ ∆G⁰=-247500+55.85T keep pO₂=10⁻¹⁰ atm, ask pH₂O/pH₂=? Kₚ=pH₂O/(pH₂ pO₂½), Kₚ=e-∆G⁰/RT=3.521∙10³ → pH₂O/pH₂=0.03521, pH₂=0.966 atm, pH₂O=0.034 atm logpH₂O=-2900/T-4.65logT+19.732, if pH₂O=0.0352 at T=27℃ CO₍g₎ + ½O₂₍g₎ = CO₂₍g₎ ∆G⁰=-282400+86.81T when T=1000K, P=1 atm and pO₂=10⁻²⁰ atm, ask pCO₂/pCO=? Sol: Kₚ=pCO₂/(pCOpO₂½), Kₚ=e-∆G⁰/RT=1.646∙10¹⁰ → pCO₂/pCO=1.646 實務上用gas flow(flowmeter or MFC)去控制壓力比值, pf: before: nᴬ=Pa/RT, nᴮ=Pb/RT, mixing: a+b=V, pᴬ=nᴬRT/V=a/(a+b), pᴮ=nᴮRT/V=b/(a+b) → pᴬ/pᴮ=a/b Ex-1. P₄₍g₎ → 2P₂₍g₎ ∆G⁰=225400+7.90TlnT-209.4T Q1: What is T for equil. with P=1 atm and XP₄=XP₂=0.5 Kₚ=pP₂²/pP₄=(XP₂²/XP₄)P=0.5, -∆G⁰/RT=lnKₚ, -27109/T-0.95lnT+25.873=0, T=1429K Q2: What is P for equil. with T=2000K atm and XP₄=XP₂=0.5 Kₚ=pP₂²/pP₄=(XP₂²/XP₄)P, Kₚ=e-∆G⁰/RT=81.83 → 81.83=0.5P, P=163.66 atm
Ex-2. Decompose of NH₃: 2NH₃₍g₎ = N₂₍g₎ + 3H₂₍g₎ ∆G⁰=87030-25.8TlnT-31.7T J Q1: final pᵢ=? at T=400℃, P=1 atm Kₚ≡pN₂pH₂³/pNH₃², Kₚ=e-∆G⁰/RT=4748
pN₂=xP/(1+2x), pH₂=3xP/(1+2x), pNH₃=(1-2x)P/(1+2x)代入Kₚ, Kₚ=27x⁴P²/[(1+2x)²(1-2x)²] → Kₚ½=5.196x²P/[(1+2x)(1-2x)]代入Kₚ=4748, P=1 → x=4945 → pN₂, pH₂, pNH₃ Q2: final pᵢ=? at T=400℃, and fixed volume fixed V, P(=1 atm) → P V=RT/P=nᴛ RT/P ⸫P=1+2x atm ⸪ Kₚ½=5.196x²P/[(1+2x)(1-2x)] 代入Kₚ=4748, P=1+2x x=0.4909, P=1.981 atm pN₂=0.4954 atm, pH₂=1.4862 atm, pNH₃=0.0092 atm Q3: Synthesis of NH₃ with H₂, N₂; let pH₂/pN₂=a, ask a=? when pNH₃ at max. 解讀:pNH₃ at max.等同dpNH₃ /da=0, 求a=? P=1 atm, P=pN₂+pH₂+pNH₃=(a+1)pN₂+pNH₃ → pN₂=(1-pNH₃)/(a+1), pH₂=a(1-pNH₃)/(a+1) Kₚ≡pNH₃²/pN₂pH₂³=pNH₃²(a+1)⁴/[a³(1-pNH₃)⁴] → Kₚa³(1-pNH₃)⁴=pNH₃²(a+1)⁴ 取ln → lnKₚ+3lna+4ln(1-pNH₃)=2lnpNH₃+4ln(a+1) ∂a → 3da/a-[4/(1-pNH₃)]dpNH₃ =(2/pNH₃)dpNH₃+4da/(a+1) → [4/(1-pNH₃)+(2/pNH₃)]dpNH₃ =[3/a-4/(a+1)]da ⸪dpNH₃ /da=0 ⸫3/a-4/(a+1)=0, a=3 Ex-3. 1 mole CH₄ + 1 mole CO₂ at T=1000K, P=1atm Final equilibrium composition ? pᵢ=? Given (1) CH₄(g)= C(s)+ 2H₂(g) ∆G₁⁰=69120-22.25TlnT+65.35 (2) 2C(s)+ O₂(g)= 2CO(g) ∆G₂⁰=-223400-175.3T (3) C(s)+ O₂(g) = CO₂(g) ∆G₃⁰=-394100-0.8T (4) H₂(g)+½O₂(g)= H₂O(g) ∆G₄⁰=-246400+54.8T sol: CH₄₍g₎ + CO₂₍g₎ = 2H₂₍g₎ + 2CO₍g₎...(5) ini 1 1 0 0 Kₚ₅= pCO²pH₂²/pCH₄pCO₂ f 1-x 1-x 2x 2x f 1-x 1-x-y 2x+y 2x-y ∆G₅⁰=(1)+(2)-(3)=239820-22.25TlnT-174.5T+65.35=15.95
H₂₍g₎ + CO₂₍g₎ = H₂O₍g₎ + CO₍g₎....(6) ∆G₆⁰=(4)-(3)+½(2)=36000-32.05T=0.62 ini 2x 1-x 0 2x Kₚ₆=pCOpH₂O/pH₂pCO₂ f 2x-y 1-x-y y 2x+y →影響(5), (5)(6)兩反應需同時平衡 nᴛ=nCH₄+nCO₂+nH₂+nCO+nH₂O=(1-x)+(1-x-y)+(2x-y)+(2x+y)+y=2(1+x) Kₚ₅= pCO²pH₂²/pCH₄pCO₂=[(2x+y)²(2x-y)²P²/(1-x)(1-x-y)(2+2x)²]=15.95 Kₚ₆=pCOpH₂O/pH₂pCO₂=[(2x+y)y/(1-x-y)(2x-y)]=0.62 聯立解 x=0.785, y=0.0775 → pCH₄=0.0602, pCO₂=0.0385, pH₂=0.4181, pCO=0.4615, pH₂O=0.0217 CO₍g₎ + ½O₂₍g₎ = CO₂₍g₎....(7) ∆G₇⁰=(3)-½(2)=-282400+86.85T Kₚ₇=exp(-∆G₇⁰/RT)=exp(369250/8314.4)=1.64∙10¹⁰=pCO₂/pCOpO₂½ pO₂½=0.0385/(0.4615∙1.64∙10¹⁰) ⸫pO₂=2.5876∙10⁻²³ atm H₂₍g₎ + ½O₂₍g₎ = H₂O₍g₎....(4) ∆G₄⁰=-246400+54.8T Kₚ₄=exp(-∆G₄⁰/RT)=exp(191600/8314.4)=1.02∙10¹⁰=pH₂O/pH₂pO₂½ pO₂½=0.0217/(0.4181∙1.02∙10¹⁰) ⸫pO₂=2.5892∙10⁻²³ atm 整個平衡狀態包括(4),(5),(6),(7)的反應, 最後平衡成分: pCH₄=0.0602, pCO₂=0.0385, pH₂=0.4181, pCO=0.4615, pH₂O=0.0217, pO₂=2.6∙10⁻²³ atm
|
|
| ( 知識學習|隨堂筆記 ) |




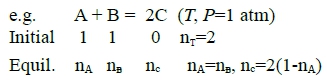
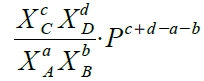 , Kₓ≡XᶜᶜXᴅᵈ/(XᴀᵃXᴃᵇ) ⸫Kₚ= Kₓ∙Pᶜ⁺ᵈ⁻ᵃ⁻ᵇ
, Kₓ≡XᶜᶜXᴅᵈ/(XᴀᵃXᴃᵇ) ⸫Kₚ= Kₓ∙Pᶜ⁺ᵈ⁻ᵃ⁻ᵇ