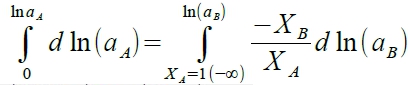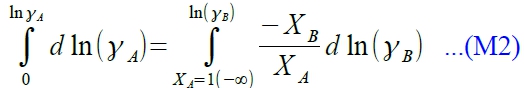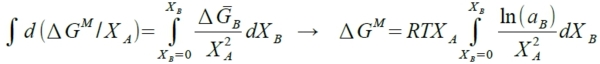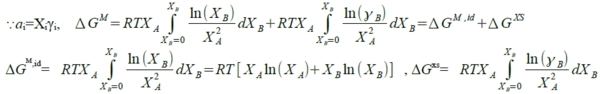字體:小 中 大
字體:小 中 大 |
|
|
|
| 2020/09/11 15:14:14瀏覽1592|回應0|推薦0 | |
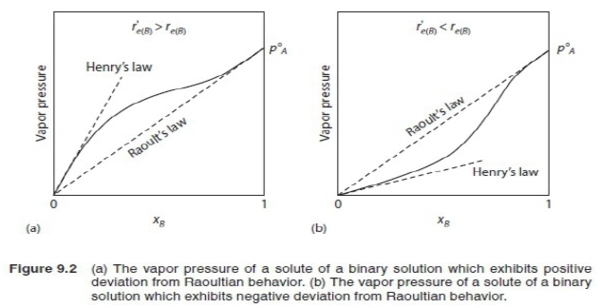
ch. 9 solution behavior *gas mixing(ideal gas): 原子間距離遠,無相互作用力的問題; 100%混合無溶解度的問題 *condensed phase(liquid or solid): strong interaction between atoms, solubility issues in solid: 1. atom size difference in crystal(尺寸影響, Hume-Rothery rule). 2. 鍵結影響: 陰電性(從外抓電子的能力), 價數valence. §Raoults law & Henrys law 1. pure liquid A: evaporation rate rₑ(A) and condensation rate rᶜ(A)=kpᴀ⁰ at equilibrium, rₑ(A)=rᶜ(A) → rₑ(A)=kpᴀ⁰ 2. pure liquid B: evaporation rate rₑ(B) and condensation rate rᶜ(B)=kpᴃ⁰ at equilibrium, rₑ(B)=rᶜ(B) → rₑ(B)=kpᴃ⁰ 3. B+(very small amount A): condensation rate of A rᶜ(A)=kpᴀ, condensation rate of B rᶜ(B)=kpᴃ Assuming a. Eᴀᴀ=Eᴃᴃ=Eᴀᴃ(bonding energy) i.e. Eᵢʲ<0 b. surface composition=bulk composition, c.surface fraction of A=Xᴀ evaporation rate of A: rₑ(A)Xᴀ; as equilibrium, rₑ(A)Xᴀ=kpᴀ ⸪rₑ(A)=kpᴀ⁰ → Xᴀpᴀ⁰=pᴀ ⸫ Xᴃpᴃ⁰=pᴃ Xᴀ=pᴀ/pᴀ⁰, Xᴃ=pᴃ/pᴃ⁰....Raoults law 4. if Eᴀᴃ is more negative(|Eᴀᴃ|>|Eᴀᴀ| and |Eᴀᴃ|>|Eᴃᴃ|), evaporation rate of A, rₑ(A)<rₑ(A) rₑ(A)Xᴀ=kpᴀ → 同除以rₑ(A)=kpᴀ⁰ → rₑ(A)Xᴀ/rₑ(A)=kpᴀ/kpᴀ⁰ → [rₑ(A)/rₑ(A)]Xᴀ=pᴀ/pᴀ⁰ → γᴀXᴀ=pᴀ/pᴀ⁰ (γᴀ<1) *γᴀ is constant, independent of Xᴀ(與成分無關)...Henrys law pᴀ/pᴀ⁰=γᴀXᴀ, pᴃ/pᴃ⁰=γᴃXᴃ
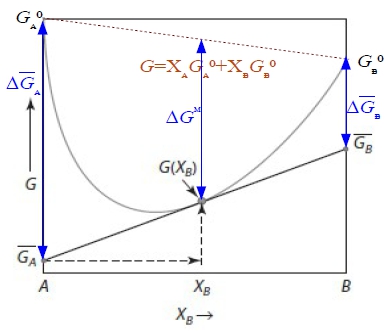
§activity of a component in solution: aᵢ≡fᵢ/fᵢ⁰, if vapor as ideal gas, fᵢ=pᵢ ⸫ aᵢ≡pᵢ/pᵢ⁰
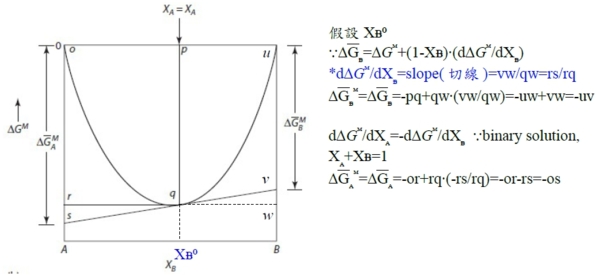
solution: (aᵢ/Xᵢ)≡γᵢ activity coeff. Raoults law: aᵢ=Xᵢ (or γᵢ=1) Henrys law: 1. aᵢ=γᵢXᵢ, γᵢ≠1 2. γᵢ independent of Xᵢ(有限範圍內有效,若超過臨界值,A原子周圍就不再都是B原子,與成分有關) §Gibbs-Duhem eq. Q=Q(T,P,nᵢ,nʲ,nₖ,....) in a solution composed of (nᵢ,nʲ,nₖ,....) →dQ=(∂Q∕∂T)ᴘ,ₙᵢ,...dT+(∂Q∕∂P)ᴛ,ₙᵢ,...dP+(∂Q∕∂nᵢ)ᴛ,ᴘ,ₙʲ,...dnᵢ+... at fixed T,P dQ=Qᵢdnᵢ+Qʲdnʲ+Qₖdnₖ+... Q=nᵢQᵢ+nʲQʲ+nₖQₖ+... → dQ=Qᵢdnᵢ+Qʲdnʲ+Qₖdnₖ+...+(nᵢdQᵢ+nʲdQʲ+nₖdQₖ+...) ∑nᵢdQᵢ=0, ∑XᵢdQᵢ=0...Gibbs-Duhem eq. §Gibbs free energy of binary solution 1. molar Gibbs free energy (G) ← solution(given) partial Gibbs free energy Gᴀ, Gᴃ ← each component=? ⸪G=XᴀGᴀ+Xᴃ Gᴃ...(1) dG=GᴀdXᴀ+ GᴃdXᴃ+XᴀdGᴀ+Xᴃ dGᴃ → dG=GᴀdXᴀ+ GᴃdXᴃ binary Xᴀ+Xᴃ =1, dXᴀ=-dXᴃ ⸫dG=(Gᴀ- Gᴃ)dXᴀ , Gᴀ- Gᴃ=dG/dXᴀ ...(2) (2)∙Xᴃ+(1) → G+Xᴃ(dG/dXᴀ)=(Xᴀ+Xᴃ ) Gᴀ → Gᴀ=G+(1-Xᴀ)(dG/dXᴀ) or Gᴃ=G+(1-Xᴃ)(dG/dXᴃ) Gᵢ=G+(1-Xᵢ)(dG/dXᵢ) 知其中之一即可求其餘自由能! e.g. G=aXᴀXᴃ, Gᴀ=? G=aXᴀ(1-Xᴀ)=aXᴀ-aXᴀ², dG/dXᴀ=a-2aXᴀ ⸫ Gᴀ=aXᴀXᴃ+Xᴃ(a-2aXᴀ)= aXᴃ-aXᴀXᴃ=aXᴃ(1-Xᴀ)=a(1-Xᴀ)² fixed T,P G=G(Xᴀ,Xᴃ )=G(Xᴀ)=a+bXᴀ+cXᴀ²+... 2. ∆Gᴹ, ∆Gᴀᴹ, ∆Gᴃᴹ i: component 1 mole i(pᵢ⁰) → mixed into solution(pᵢ), ∆Gᵢ=Gᵢ₍ₛₒₗ₎-Gᵢ₍ₚᵤᵣₑ₎=RTln(pᵢ/pᵢ⁰)=RTlnaᵢ note: Gᵢ₍ₛₒₗ₎=Gᵢ, Gᵢ₍ₚᵤᵣₑ₎=Gᵢ⁰, ⸫∆Gᵢ=∆Gᵢ=Gᵢ-Gᵢ⁰=RTlnaᵢ A: nᴀ and B: nᴃ mixing at (T,P), 混合前: Gᵇᵉᶠ=nᴀGᴀ⁰+nᴃGᴃ⁰, 混合後: Gₐᶠₜ=nᴀGᴀ+nᴃGᴃ ⸫∆Gᴹ=Gₐᶠₜ-Gᵇᵉᶠ= nᴀ(Gᴀ-Gᴀ⁰)+nᴃ(Gᴃ-Gᴃ⁰)=nᴀ∆Gᴀ+nᴃ∆Gᴃ=nᴀRTlnaᴀ+nᴃRTlnaᴃ=RT(nᴀlnaᴀ+nᴃlnaᴃ) → ∆Gᴹ=RT(Xᴀlnaᴀ+Xᴃlnaᴃ) 除以nᴀ+nᴃ成為molar Gibbs free energy. or 直接寫 ∆Gᴹ=XᴀRTlnaᴀ+XᴃRTlnaᴃ 3. 圖解 Gᵢ(Gᴀ,Gᴃ), ∆Gᵢ(∆Gᴀ,∆Gᴃ) given: ∆Gᴹ curve, ask ∆Gᴀ=?, ∆Gᴃ=?
Given G₍ₛₒₗ₎, curve, ask Gᴀ=?, Gᴃ=?
§properties of ideal solution ideal solution: Eᴀᴀ=Eᴃᴃ=Eᴀᴃ(same bonding energy), aᵢ=Xᵢ (or γᵢ=1) ⸪∆Gᵢ=RTlnaᵢ ⸫∆Gᵢ=RTlnXᵢ ask ∆Gᴹ=?, ∆Hᴹ=? ∆Sᴹ=?, ∆Vᴹ=? 與ideal das 混合結果相同 ∆Gᴹ=RT∑XᵢlnXᵢ, ∆Sᴹ=- R∑XᵢlnXᵢ, ∆Hᴹ=0, ∆Vᴹ=0 ∆Vᴹ=∑Xᵢ∆Vᵢ 由G求V→ dG=-SdT+VdP+∑ μᵢdnᵢ at fixed T, (∂G∕∂P)ᵀ,ᶜₒₘₚ=V (ideal gas) 沿用至ideal solution, 混合後: Vᵢ=(∂Gᵢ∕∂P)ᵀ,ᶜₒₘₚ, 混合前: Vᵢ⁰=(∂Gᵢ⁰∕∂P)ᵀ ∆Vᵢ=Vᵢ-Vᵢ⁰=[∂(Gᵢ-Gᵢ⁰)∕∂P]ᵀ,ᶜₒₘₚ=[∂(RTlnXᵢ)∕∂P]ᵀ,ᶜₒₘₚ=0, Vᵢ=Vᵢ⁰ → ∆Vᴹ=0 ∆Hᴹ=∑Xᵢ∆Hᵢ 由G求H→ Gibbs-Hlemholtz eq. 混合後: [∂(Gᵢ/T)∕∂T]ᴾ,ᶜₒₘₚ=-Hᵢ/T², 混合前: [∂(Gᵢ⁰/T)∕∂T]ᴾ,ᶜₒₘₚ=-Hᵢ⁰/T² →
∆Hᴹ=0
∆Sᴹ=? ∆Gᴹ=∑Xᵢ∆Gᵢ=RT∑XᵢlnXᵢ, ∆Gᴹ=∆Hᴹ-T∆Sᴹ, ∆Sᴹ=-∆Gᴹ/T=-R∑XᵢlnXᵢ 由G求S→ -(∂G∕∂T)ᴾ,ᶜₒₘₚ=S → -(∂∆Gᵢ∕∂T)ᴾ,ᶜₒₘₚ=∆Sᵢ=-RlnXᵢ, ∆Sᴹ=∑Xᵢ∆Sᵢ=-R∑XᵢlnXᵢ 以binary solution為例,nᴀ mole A與nᴃ mole B 混合, 混合前entropy=S⁰, 混合後entropy=S ∆Sᴹ=S-S⁰=klnΩ-klnΩ⁰=kln[(nᴀ+nᴃ)!/(nᴀ!nᴃ!)]-kln(nᴀ!/nᴀ!∙nᴃ!/nᴃ!) i.e. 統計熱力 S≡klnΩ, stirling approx. LnN!≈NlnN-N → ∆Sᴹ=k[(nᴀ+nᴃ)ln(nᴀ+nᴃ)-(nᴀ+nᴃ)-nᴀlnnᴀ+nᴀ-nᴃlnnᴃ +nᴃ] ″configuration entropy″ =-k{nᴀln[nᴀ/(nᴀ+nᴃ)]+nᴃln[nᴃ /(nᴀ+nᴃ)]}=-k[nᴀlnXᴀ+nᴃlnXᴃ]=-k(nᴀ+nᴃ)[XᴀlnXᴀ+XᴃlnXᴃ] =-k∙Nᴀ∙n(XᴀlnXᴀ+XᴃlnXᴃ)=-R∙n(XᴀlnXᴀ+XᴃlnXᴃ) → ∆Sᴹ=-R(XᴀlnXᴀ+XᴃlnXᴃ), ⸫∆Sᴹ=-R∑XᵢlnXᵢ e.g. Vacancy or impurity, 從自由能看平衡濃度, ∆Gᴹ=∆Hᴹ-T∆Sᴹ, 焓視為形成空位所必需的能量,另一方面空位加入晶格中(如二元溶液混合)造成亂度增加,最後∆Gᴹ=0達到平衡 equilibrium NV =Ne⁻Qᵛ/ᴿᵀ, k=-1.38∙10⁻²³ J/K Boltzmanns constant i.e. N: total # of atomic site, Qv: energy required for the formation of a vacancy.
§non-ideal solution, γᵢ≡aᵢ/Xᵢ, γᵢ≠1(γᵢ>1 or γᵢ<1) → γᵢ=f(Xᵢ,T) ⸪∆Gᵢ=RTlnaᵢ=RTlnXᵢ+RTlnγᵢ → ∆Hᵢ≠0 ∆Gᵢ=RTlnaᵢ → 求∆Hᵢ [∂(∆Gᵢ/T)∕∂T]ᴾ,ᶜₒₘₚ=-∆Hᵢ/T² → [∂(Rlnγᵢ)∕∂T]ᴾ,ᶜₒₘₚ=-∆Hᵢ/T² → [∂(Rlnγᵢ)∕∂(1/T)]ᴾ,ᶜₒₘₚ=-∆Hᵢ notes: case #1. γᵢ≠f(T), independent of T. ∆Hᵢ=0 case #2. in general, γᵢ=f(T) e.g. If γᴀ>1 in binary solution, γᴀ≡[rₑ(A)/rₑ(A)]表示|Eᴀᴃ|<|Eᴀᴀ|, A-B bonding比較弱,solute A容易蒸發; 另外任何溶液溫度升高,亂度加大,使 γᵢ→1, ⸫dγᵢ/dT<0, dlnγᵢ/dT<0. Case #3. If γᵢ>1, 溫度升高使 γᵢ→1, ⸫dγᵢ/dT<0, dlnγᵢ/dT<0 → ∆Hᵢ>0 endothermic reaction, |Eᴀᴃ|<|Eᴀᴀ|, clustering case #4. If γᵢ<1, 溫度升高使 γᵢ→1, ⸫dγᵢ/dT>0, dlnγᵢ/dT>0 → ∆Hᵢ<0 exothermic reaction, |Eᴀᴃ|>|Eᴀᴀ|, ordering to form compound. §application of Gibbs-Duhem eq. 1. given aᴃ(Xᴃ) → calculate aᴀ(Xᴀ) 2. if solute follows Henrys law, solvent would follows Raoults law. The same result in the opposite way. 3. given aᴀ(Xᴀ) → calculate ∆Gᴹ=? ∑XᵢdQᵢ=0...Gibbs-Duhem eq. ⸫∑XᵢdGᵢ=0 → RT∑Xᵢdlnaᵢ=0 binary solution, Xᴀdlnaᴀ+Xᴃdlnaᴃ=0 → dlnaᴀ=-(Xᴃ/Xᴀ)dlnaᴃ,
Xᴀdlnaᴀ+Xᴃdlnaᴃ=0 → XᴀdlnXᴀ+Xᴀdlnγᴀ+XᴃdlnXᴃ+Xᴃdlnγᴃ=0 ⸪ XᴀdlnXᴀ+XᴃdlnXᴃ=Xᴀ(dXᴀ/Xᴀ)+Xᴃ(dXᴃ/Xᴃ)=dXᴀ+dXᴃ=0, ⸫Xᴀdlnγᴀ+Xᴃdlnγᴃ=0 → dlnγᴀ=-(Xᴃ/Xᴀ)dlnγᴃ→
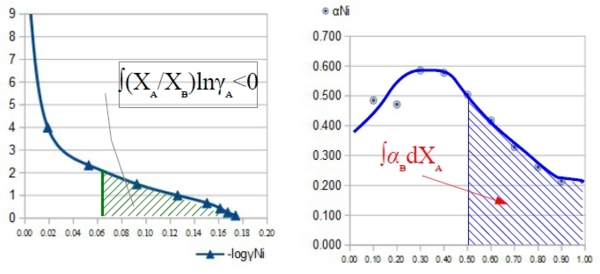
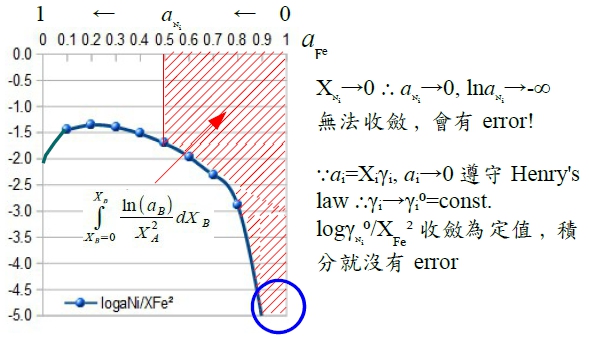
define: α function, αᵢ≡lnγᵢ/(1-Xᵢ)²
when Xᴃ→1 or Xᴀ→0 ⸫γᴃ→1, lnγᴃ=0; 1-Xᴃ→0, αᴃ=lnγᴃ/(1-Xᴃ)²→finite value lnγᴀ= -XᴀXᴃ αᴃ-∫Xᴀ=1αᴃdXᴀ...(M3) pf: αᴃ=lnγᴃ/(1-Xᴃ)² → lnγᴃ= αᴃ Xᴀ² → dlnγᴃ=2αᴃ XᴀdXᴀ+Xᴀ²dαᴃ 代入dlnγᴀ=-(Xᴃ/Xᴀ)dlnγᴃ 式中,
dlnγᴀ=-(Xᴃ/Xᴀ)∙(2αᴃ XᴀdXᴀ+Xᴀ²dαᴃ)=-2αᴃ Xᴃ dXᴀ-Xᴀ Xᴃ dαᴃ d(αᴃXᴀ Xᴃ)=Xᴀ Xᴃ dαᴃ+αᴃ XᴃdXᴀ+αᴃ XᴀdXᴃ 移項代入上式 dlnγᴀ=-2αᴃ Xᴃ dXᴀ-d(αᴃXᴀ Xᴃ)+αᴃ XᴃdXᴀ+αᴃ Xᴀ dXᴃ=-d(αᴃXᴀ Xᴃ)-αᴃ (XᴃdXᴀ-XᴀdXᴃ) ⸪ dXᴀ=-dXᴃ dlnγᴀ=-d(αᴃXᴀ Xᴃ)-αᴃdXᴀ → 積分即得
3. 已知aᴃ, ∆Gᴃ=RTlnaᴃ, 求∆Gᴹ=? ⸪ ∆Gᴃ=∆Gᴹ+(1-Xᴃ)∙(d∆Gᴹ/dXᴃ ) → ∆GᴃdXᴃ=∆GᴹdXᴃ +(1-Xᴃ)d∆Gᴹ → ∆GᴃdXᴃ=-∆GᴹdXᴀ+Xᴀd∆Gᴹ 除以Xᴀ² → ∆GᴃdXᴃ/Xᴀ²=(-∆GᴹdXᴀ+Xᴀd∆Gᴹ)/Xᴀ²=d(∆Gᴹ/Xᴀ) →積分 →
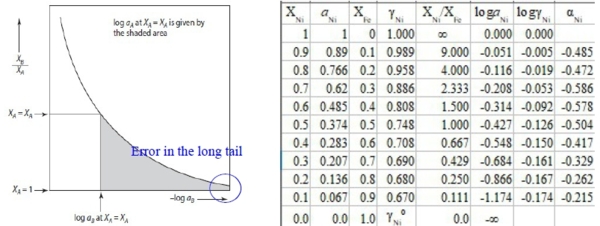
Ex. Fe-Ni, aᴺᵢ; Fe-Cu, aᴄᵤ
2. solute B follows Henrys law, solvent A will follow Raoults law. γᴃ=γᴃ⁰=constant, pf: ⸪aᴃ=Xᴃγᴃ⁰, ⸫lnaᴃ =lnXᴃ+lnγᴃ⁰ → dlnaᴃ =dlnXᴃ ; G-D eq. dlnaᴀ=-(Xᴃ/Xᴀ)dlnaᴃ → dlnaᴀ=-(Xᴃ/Xᴀ)dlnXᴃ=-(Xᴃ/Xᴀ)∙(dXᴃ /Xᴃ)=-dXᴃ /Xᴀ=dXᴀ /Xᴀ=dlnXᴀ → 積分 ∫dlnaᴀ=∫dlnXᴀ lnaᴀ=lnXᴀ , aᴀ=Xᴀ 反向也可證明 §Regular solution *ideal: aᵢ=Xᵢ or γᵢ=1, ∆Hᵢ=0, ∆Sᵢ=-RlnXᵢ, ∆Gᵢ=RTlnXᵢ *non-ideal: γᵢ≠1 and γᵢ≠const. γᵢ=f(Xᵢ,T) → ∆Hᵢ=-T²[∂(Rlnγᵢ)∕∂T]ᴾ,ᶜₒₘₚ≠0=f(Xᵢ,T) 太複雜了! 找一個simplest function of ∆Hᵢ → Regular solution! Define „regular solution” in mathematic form: 1. ∆Sᵢ=∆Sᵢid =-RlnXᵢ, 2. ∆Hᵢ=f(Xᵢ) only! Indep. of T. ∆Hᴹ=∑Xᵢ∆Hᵢ=f"(Xᵢ), 特性: 當Xᴀ→0, Xᴃ→1 or Xᴀ→1, Xᴃ→0時, ∆Hᴹ→0 ⸫∆Hᴹ=XᴀXᴃ(α+bXᴀ +cXᴀ²+...), simplest form: ∆Hᴹ=XᴀXᴃα 求∆Hᴀ=?, ∆Hᴃ=? ∆Hᵢ=∆Hᴹ+(1-Xᵢ)(d∆Hᴹ/dXᵢ), ∆Hᴹ=αXᴀ(1-Xᴀ)=αXᴀ-αXᴀ² ⸫∆Hᴀ=XᴀXᴃα+(1-Xᴀ)(α-2αXᴀ)=XᴀXᴃα+Xᴃ(α-2αXᴀ)=αXᴃ-αXᴀ Xᴃ=αXᴃ(1-Xᴀ)=αXᴃ² ∆Hᴀ=α(1-Xᴀ)², ∆Hᴃ=α(1-Xᴃ)², ∆Gᴀ=∆Hᴀ-T∆Sᴀ=RTlnaᴀ=RTlnXᴀ+RTlnγᴀ=RTlnγᴀ-T(-RlnXᴀ) ⸫∆Hᴀ=RTlnγᴀ 前面define: α function, αᵢ≡lnγᵢ/(1-Xᵢ)² → αᴀ=lnγᴀ/(1-Xᴀ)² αᴀ=lnγᴀ/(1-Xᴀ)²=1/(1-Xᴀ)²∙[α(1-Xᴀ)²/RT]=α/RT ⸪∆Hᴀ=α(1-Xᴀ)²=RTlnγᴀ 同時αᴃ= α/RT=αᴀ=α → α=αRT 物理意義? §Excess quantity, „XS” ∆Sᵢₓₛ, ∆Hᵢₓₛ, ∆Gᵢₓₛ ∆Gᵢ=RTlnaᵢ=RTlnXᵢ+RTlnγᵢ=∆Gᵢid+∆Gᵢₓₛ, ∆Gᴹ=∑Xᵢ∆Gᵢ=∆Hᴹ-T∆Sᴹ ⸫∆Gₓₛ=∆Gᴹ-∆Gᴹ,id=(∆Hᴹ-∆Hᴹ,id)-T(∆Sᴹ-∆Sᴹ,id) ⸪∆Hᴹ,id=0, ∆Sᴹ=∆Sᴹ,id →∆Gₓₛ=∆Hᴹ, ∆Gₓₛ=RT(Xᴀlnγᴀ+Xᴃlnγᴃ)=∆Hᴹ, ∆Gₓₛ= RT∑ Xᵢlnγᵢ Regular solution: αᴃ=αᴀ=α, lnγᴀ=α(1-Xᴀ)², lnγᴃ=α(1-Xᴃ)²代入上式∆Gₓₛ ⸫∆Gₓₛ=∆Hᴹ=RTαXᴀXᴃ=αXᴀXᴃ indep. of T. ∆Sₓₛ=0, ∆Sₓₛ=-(∂∆Gₓₛ∕∂T)ᴾ,ᶜₒₘₚ=0 *At a fixed composition, ∆Gᴀₓₛ=RTlnγᴀ → RT₁lnγᴀ₁=RT₂lnγᴀ₂ → lnγᴀ₁/lnγᴀ₂=T₂/T₁ Summary for simplest regular solution: ∆Sᵢₓₛ=0, ∆Hᵢₓₛ=RTlnγᵢ, ∆Gᵢₓₛ=RTlnγᵢ 1. ∆Gₓₛ=∆Hᴹ=RTαXᴀXᴃ=αXᴀXᴃ 2. ∆Gᵢₓₛ=∆Hᵢₓₛ=RTlnγᵢ 3. ∆Sᵢₓₛ=0, ∆Sₓₛ=0, ∆Sᵢ=∆Sᵢid =-RlnXᵢ 4. αᴃ=αᴀ=α=α/RT 實例判斷: 1. if ∆Hᴹ=bXᴀXᴃ, ∆Gₓₛ=bXᴀXᴃ, b≠b→ ∆Gₓₛ≠∆Hᴹ non-regular! e.g. Au-Cu at 1550K, ∆Gₓₛ=-24060XAuXCu and ∆Hᴹ non-symmetric → non-regular! Au-Ag at 1350K, ∆Hᴹ=-20590XAuXAg and ∆Gₓₛ non-symmetric → non-regular! 2. T=800K,
§9-11 sub-regular solution
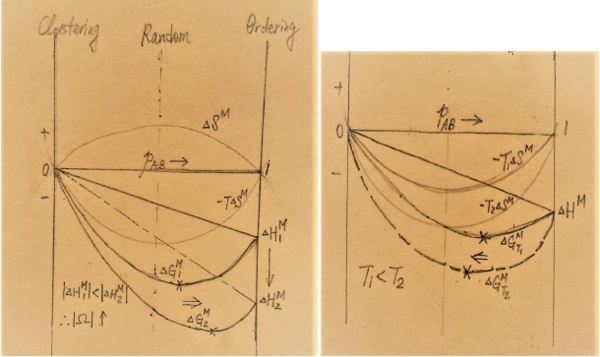
∆Gₓₛ=∆Hᴹ=XᴀXᴃ(α+bXᴀ)≠f(T), e.g. Au-Ag at 1350K, ∆Gₓₛ=∆Hᴹ=XᴀXᴃ(α+bXᴀ ), α=-11320J, b=1940J *non-regular: ∆Gₓₛ=(a+bXᴀ)XᴀXᴃ∙(1-T/τ)=f(T), ∆Sₓₛ=-(∂∆Gₓₛ∕∂T)ᴾ,ᶜₒₘₚ=1/τ(a+bXᴀ)XᴀXᴃ≠0 ∆Hᴹ=∆Hₓₛ=∆Gₓₛ+T∆Sₓₛ=(a+bXᴀ)XᴀXᴃ→ ∆Gₓₛ≠∆Hᴹ non-regular! §Quasi-chemical model of solution, 以統計方法解釋α的物理意義 e.g. Binary solution, A+B, Nᴀ atoms and Nᴃ atoms(Nᴀ+Nᴃ=N₀=6.02∙10²³) 考慮 ∆Hᴹ=∆Uᴹ+P∆Vᴹ, assume ∆Vᴹ=0, ⸪Vᴀ⁰=Vᴀ=Vᴃ= Vᴃ⁰; ∆Hᴹ=∆Uᴹ=∆Eᴹ (鍵結能變化) 鍵結能, E=ꝑᴀᴀEᴀᴀ+ꝑᴃᴃEᴃᴃ+ꝑᴀᴃEᴀᴃ, i.e. Eᵢʲ: bonding energy of i-j, ꝑᵢʲ: # of i-j bonds 假設A原子的配位數為Zᴀ, Nᴀ原子的鍵結數可表示成: Nᴀ∙Zᴀ=2ꝑᴀᴀ+ꝑᴀᴃ → ꝑᴀᴀ=½(Nᴀ∙Zᴀ-ꝑᴀᴃ), 同理 B原子的配位數設為Zᴃ, Nᴃ原子的鍵結數可表示成: Nᴃ∙Zᴃ=2ꝑᴃᴃ+ꝑᴃᴀ → ꝑᴃᴃ=½(Nᴃ∙Zᴃ-ꝑᴃᴀ) 代入鍵結能式中, E= ½Nᴀ∙ZᴀEᴀᴀ+½Nᴃ∙ZᴃEᴃᴃ+ꝑᴀᴃ[Eᴀᴃ- ½(Eᴀᴀ+Eᴃᴃ)] Pure A: Nᴀ∙Zᴀ=2ꝑᴀᴀ, Eᴀ⁰=ꝑᴀᴀEᴀᴀ= ½Nᴀ∙ZᴀEᴀᴀ; Pure B: Nᴃ∙Zᴃ=2ꝑᴃᴃ, Eᴃ⁰=ꝑᴃᴃEᴃᴃ=½Nᴃ∙ZᴃEᴃᴃ ∆Eᴹ=E-(Eᴀ⁰+Eᴃ⁰)=ꝑᴀᴃ[Eᴀᴃ- ½(Eᴀᴀ+Eᴃᴃ)] Assume Zᴀ=Zᴃ=Z, ꝑᴀᴃ=? 在計算ꝑᴀᴃ之前, ⸪regular solution的定義: ∆Sᵢ=∆Sᵢid =-RlnXᵢ, 表示溶液處於complete random的條件下, 原子間鍵結沒有偏好。換言之,某一固定溫度的熱能足以彌補原子間鍵結能的差異,還是處於complete random. 例如: Arrehnius eq. D=D₀e⁻q/ᵏᵀ= D₀e⁻Q/ᴿᵀ 視為粒子的活化能q與kT做比較或Q與平均熱能RT比較,得到的平衡量。因此|∆Hᴹ|≤RT視為溶液處於complete random ∆Hᴹ≈∆Eᴹ=ꝑᴀᴃ[Eᴀᴃ- ½(Eᴀᴀ+Eᴃᴃ)] 考慮A-B鍵數, 相鄰位置A-B配對的機率: ℓᴀᴃ=2XᴀXᴃ total # of bond=½N₀∙Z, ⸫ ꝑᴀᴃ=½N₀∙Zℓᴀᴃ =N₀∙ZXᴀXᴃ ∆Hᴹ=XᴀXᴃ∙N₀Z[Eᴀᴃ- ½(Eᴀᴀ+Eᴃᴃ)] compare with regular ∆Hᴹ=XᴀXᴃα let Ω≡N₀Z[Eᴀᴃ- ½(Eᴀᴀ+Eᴃᴃ)] 鍵結能差異 → Ω=α=RTα ∆Hᴹ=ΩXᴀXᴃ=∆Gₓₛ, ∆Hᵢ=? ∆Hᴀ=Ω(1-Xᴀ)², ∆Hᴃ=Ω(1-Xᴃ)², ∆Sᴀ=-RlnXᴀ, ∆Sᴃ=-RlnXᴃ ∆Gᴀ=∆Hᴀ-T∆Sᴀ=Ω(1-Xᴀ)²-T(-RlnXᴀ) vs. ∆Gᴀ=RTlnaᴀ=RTlnXᴀ+RTlnγᴀ ⸫ RTlnγᴀ=Ω(1-Xᴀ)² → lnγᵢ=(Ω/RT)∙(1-Xᵢ)² notes: 1. if Ω=0, Eᴀᴃ=½(Eᴀᴀ+Eᴃᴃ) → γᵢ=1 ideal solution 2. if Ω<0, ∆Hᴹ<0 exothermic, ⸪Eᵢʲ<0 ⸫ |Eᴀᴃ|>½|Eᴀᴀ+Eᴃᴃ| → γᵢ<1 negative deviation 3. if Ω>0, ∆Hᴹ>0 endothermic, ⸪Eᵢʲ<0 ⸫ |Eᴀᴃ|<½|Eᴀᴀ+Eᴃᴃ| → γᵢ>1 positive deviation 4. Henrys law: aᵢ=γᵢ⁰Xᵢ, as Xᵢ→0 γᵢ⁰=constant=eΩ/ᴿᵀ 5. regular solution重要條件需要complete random, when |Eᴀᴃ|>>½|Eᴀᴀ+Eᴃᴃ| → |Ω|鍵結能差異很大,會破壞complete random, 不適合用regular solution 模擬 6. equilibrium „configuration” of solution at fixed T, P Equlibrium means ∆Gₘᵢₙ, ∆G=∆H-T∆S regular: ∆Hᴹ=ꝑᴀᴃ[Eᴀᴃ- ½(Eᴀᴀ+Eᴃᴃ)], ꝑᴀᴃ=(½N₀∙Z)∙ℓᴀᴃ ℓᴀᴃ: probability of A-B bond ⸫∆Hᴹ=½ℓᴀᴃ Ω
1. fixed T, |Ω|增加,∆Gₘᵢₙ會向ordering移動. 2. fixed Ω, 溫度增加,∆Gₘᵢₙ會向random移動.
Ex-1. Cu-Au solid solution at 873K, regular: ∆Gₓₛ,=-28280XAuXCu J/mole. Given: pure lnpCu⁰=-40920/T-0.86lnT+21.67 pure lnpAu⁰=-45650/T-0.306lnT+10.81 ask pAu=? pCu=? at Xcu=0.6 aCu=fCu/fCu⁰=pCu/pCu⁰, aAu=pAu/pAu⁰; aᵢ=γᵢXᵢ=pᵢ/pᵢ⁰ and lnγᵢ=(Ω/RT)∙(1-Xᵢ)², 先求γAu, γCu即可得 aCu, aAu αᵢ≡lnγᵢ/(1-Xᵢ)²=Ω/RT, αᴃ=αᴀ=α=α/RT Ex-2. Ga-Cd liquid solution at 700K, regular: XGa=0.5, aGa=0.79. Ask Ega-Cd=? Given: ΔHₑᵥₐₚ,Ga=270000J, ΔHₑᵥₐₚ,Cd=100000J, ZGa=11, ZCd=8 Ω≡N₀Z[Eᴀᴃ- ½(Eᴀᴀ+Eᴃᴃ)] let A=Ga, B=Cd; ΔHₑᵥₐₚ,i=½N₀∙ZEᵢᵢ → 求Eᴀᴀ, Eᴃᴃ EGa-Ga=-8.15∙10⁻²⁰J, ECd-Cd=-4.15∙10⁻²⁰J lnγᵢ=(Ω/RT)∙(1-Xᵢ)² 從γᵢ求出Ω, 代入Ω定義式中求EGa-Cd γGa=aGa/XGa=1.39 → lnγGa=(Ω/RT)∙(1-XGa)² ⸫Ω=10745J Assume Z=½(ZCd+ZGa)=9.5 Ega-Cd=-5.96∙10⁻²⁰J |
|
| ( 知識學習|隨堂筆記 ) |



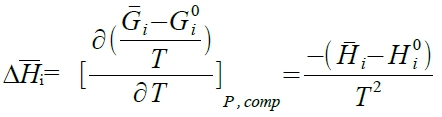 , ⸪(Gᵢ-Gᵢ⁰)/T=RlnXᵢ, ⸫∆Hᵢ=0
, ⸪(Gᵢ-Gᵢ⁰)/T=RlnXᵢ, ⸫∆Hᵢ=0