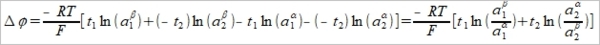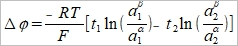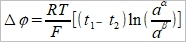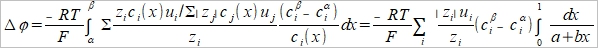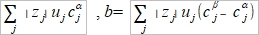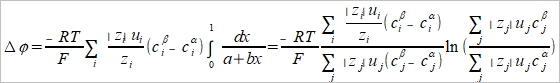字體:小 中 大
字體:小 中 大 |
|
|
|
| 2018/09/05 21:15:39瀏覽698|回應0|推薦0 | |
Chap. 3 Electrolytic conductance 電解溶液的導電性 electrical conductivity, cond.= 1/resist. Resistivity, ρ=RA/L i.e. A: cross-sction area, L: length conductivity, κ=1/ρ=L/RA....(1) Molar conductivity in electrolyte solution: Λ=κ/C C:concentration in mol m⁻³(mM) concentration dependence: Λ=Λ°-s√C Λ°:infinite dilution case, Λ°=ν₊Λ₊°+ν₋Λ₋° transference number: t₊, t₋ and t₊+t₋=1 t₊=ν₊Λ₊/Λ, t₋=ν₋Λ₋/Λ where ν₊Λ₊+ν₋Λ₋=Λ 如何去量t₊, t₋ ? cond. 與t₊, t₋有關(hittorf cell) mobility, uᵢ=|vᵢ|/|E| vᵢ: velocity, E: 電場 electric current i=ΔΦ/R, E=-ΔΦ/L and R=L/κA ⸫i=-κAE (i/A)ᵢ=CᵢzᵢFvᵢ= -κᵢE 因此 vᵢ=-κᵢE/CᵢzᵢF, uᵢ=κᵢ/Cᵢ|zᵢ|F=Λᵢ/|zᵢ|F ⸫ Λᵢ=uᵢ|zᵢ|F.....(2) Frictional coefficient, fᵢ viscous drag force Fᵢ=fᵢvᵢ, and under steady-state condition fᵢvᵢ=zᵢeE uᵢ=|vᵢ|/|E|=|zᵢ|e/fᵢ and Λᵢ=zᵢ²Fe/fᵢ Stokes radius, rᵢ, effective size 與hydrodynamicsize有關 viscosity, η fᵢ=6πηrᵢ uᵢ=|zᵢ|e/fᵢ=|zᵢ|e/6πηrᵢ → rᵢ=|zᵢ|e/6πηuᵢ 速度與rᵢ 成反比 ex. 用AC frequency適當調整, 可以使ion不受rᵢ影響conductivity, 測出真正的Λ° theoretical calculation:
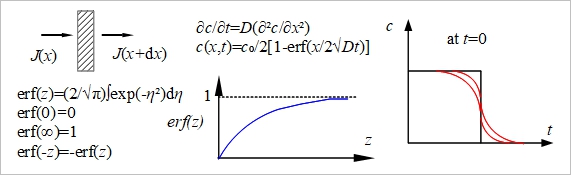
i.e. z₁, z₂: positive and negative ion charges, xᴀ: ion atnosphere thickness diffusion concentration gradient 1. Ficks 1st law, J=-D(∂c/∂x) ex. 電鍍時diffusion會影響電鍍層表面粗糙度 2. 2nd law
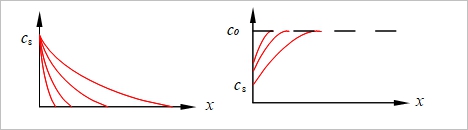
a. Carburizing, c=cₛ[1-erf(x/2√Dt)] b. De-carburizing, c=(c₀-cₛ)erf(x/2√Dt)
motion of ion μᵢ: chemical potential, ηᵢ=μᵢ+zeϕ vᵢ=-1/fᵢ(∂ηᵢ/∂x), dηᵢ=dμᵢº+RTdlnaᵢ+zᵢedϕ → vᵢ=-1/fᵢ(RTdlnaᵢ/dx+zᵢedϕ/dx) a. activity gradient=0, vᵢ= -|zᵢ|eE/fᵢ → |vᵢ|/E=|zᵢ|e/fᵢ=uᵢ b. dϕ/dx=0, E=0, vᵢ= -1/fᵢ(RTdlnaᵢ/dx)≈-1/fᵢ(RTdlncᵢ/dx)=-RT/fᵢ(1/cᵢ)(dcᵢ/dx) Jᵢ=vᵢcᵢ= -RT/fᵢ(dcᵢ/dx) ⸫Dᵢ= RT/fᵢ Gas: D≈ 10⁻¹ cm²/sec, Liq.: D≈ 10⁻⁶ cm²/sec, Solid: D≈ 10⁻⁸ cm²/sec(快熔化時) or D≈ 10⁻¹⁶ cm²/sec fᵢ=RT/Dᵢ and fᵢ=|zᵢ|e/uᵢ → RT/Dᵢ=|zᵢ|e/uᵢ Einstein relationship uᵢ=|zᵢ|eDᵢ/RT and uᵢ=Λᵢ/|zᵢ|e ⸫Dᵢ= ΛᵢRT/|zᵢ|²e² ex. Na⁺, Mg⁺⁺, Al³⁺ Λ° depend on charge on ion, D° depend on concentration of ion. Junction potential, Δϕ
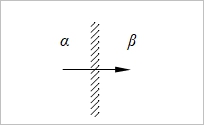
δQ=ΣFzᵢδnᵢ, tᵢ=Fzᵢδnᵢ/FΣzᵢδnᵢ=Fzᵢδnᵢ/δQ, δnᵢ=(tᵢ/zᵢ)(δQ/F) ΔG=Σdηᵢδnᵢ, ηᵢ=μᵢº+RTlnaᵢ+zᵢFϕ → dηᵢ=RTdlnaᵢ+zᵢFdϕ → ΔG=Σ(RTdlnaᵢ+zᵢFdϕ)δnᵢ=Σ(RTtᵢ/zᵢF)dlnaᵢδQ+ tᵢδQdϕ=[(RT/F)Σ(tᵢ/zᵢ)dlnaᵢ+Σtᵢdϕ]δQ When equilibrium, ΔG=0 → dϕ=-(RT/F)Σ(tᵢ/zᵢ)dlnaᵢ ⸪Σtᵢ=1 積分 Δϕ=ϕβ-ϕα=
也可寫成
if a₁α=a₂α=aα, a₁ᵝ=a₂ᵝ=aᵝ,
Henderson assumption cᵢ(x)=cᵢᵝx+cᵢα(1-x) dlnaᵢ/dx=dlncᵢ/dx=(1/cᵢ)(dcᵢ/dx) → dcᵢ/dx=cᵢᵝ- cᵢα dlnaᵢ=(dlnaᵢ/dx)dx=[(cᵢᵝ- cᵢα)/cᵢ]dx....(a) and tᵢ=zᵢcᵢ(x)uᵢ/Σ|zj|cj(x)uj.....(b) (a), (b)代入junction potential
where a=
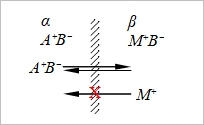
Donnan membrane
μᵢºα+RTlnaᵢα+zᵢFϕα=μᵢºᵝ+RTlnaᵢᵝ+zᵢFϕᵦ for i=1 RTlna₁α=RTlna₁ᵝ+z₁FΔϕ.....(1) if z₁=-z₂ for i=2 RTlna₂α=RTlna₂ᵝ+z₂FΔϕ.....(2) (1)+(2), RTlna₁αa₂α=RTlna₁ᵝa₂ᵝ → a₁αa₂α=a₁ᵝa₂ᵝ Δϕ=(RT/F)ln(a₁α/a₁ᵝ)=(RT/F)ln(a₂ᵝ/a₂α) if γᵢα=γᵢᵝ → c₁αc₂α=c₁ᵝc₂ᵝ 考慮電中性: in α, c₁α-c₂α=0; in β, zero-charge condition → c₁ᵝ-c₂ᵝ+zᴍcᴍ=0 |
|
| ( 知識學習|隨堂筆記 ) |



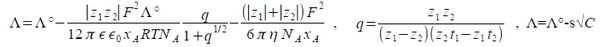
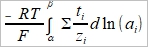 ,
,